18
Oct
Questions & answers
in
Comments
Questions & answers
Questions & answers feature on Google SERP is displayed when searches are looking for answers or solutions. Results provided in this feature are extracted from web pages that have content marked up as a question and its answers.
In DataForSEO API responses, Q&A cards of the carousel are returned as structured items – “questions_and_answers_element”.

Question text | question_text

Answer text | answer_text

Source | source
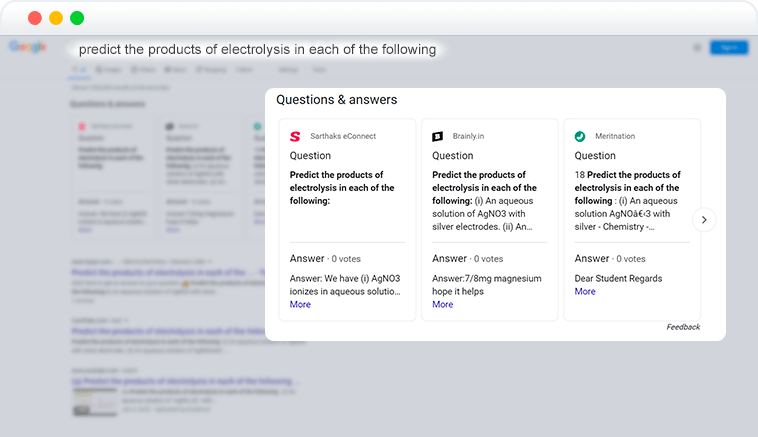
Element in SERP API
{
"type":"questions_and_answers",
"rank_group": 1,
"rank_absolute": 1,
"position":"left",
"xpath": "/html[1]/body[1]/div[7]/div[2]/div[10]/div[1]/div[2]/div[1]/div[2]/div[2]/div[1]/div[1]/div[1]",
"items":[
{
"type":"questions_and_answers_element",
"url": "https://www.sarthaks.com/8816/predict-the-products-of-electrolysis-in-each-of-the-following",
"question_text": "Predict the products of electrolysis in each of the following:",
"answer_text": "Answer: We have\n\n(i) AgNO3 ionizes in aqueous solutions to form Ag+ and NO3- ions.\n\nOn electrolysis, either Ag+ ions or H2O molecules can be reduced ...",
"source":"Sarthaks eConnect",
"domain":"www.sarthaks.com",
"votes": 0
},
{
"type":"questions_and_answers_element",
"url":"https://brainly.in/question/17093922",
"question_text":"Predict the products of electrolysis in each of the following:",
"answer_text":"Answer:7/8mg magnesium hope it helps",
"source":"Brainly.in",
"domain":"brainly.in",
"votes": 0
},
{
"type":"questions_and_answers_element",
"url": "https://www.meritnation.com/ask-answer/question/18-predict-the-products-of-electrolysis-in-each-of-th/electrochemistry/12490103",
"question_text":"Predict the products of electrolysis in each of the following",
"answer_text":"Dear Student\n\nRegards",
"source":"Meritnation",
"domain":"www.meritnation.com",
"votes": 0
},
{
"type":"questions_and_answers_element",
"url": "https://www.toppr.com/en-us/ask/question/predict-the-products-of-electrolysis-in-each-of-the-followingi-an-aqueous-solution-of-agno3/",
"question_text":"Predict the products of electrolysis",
"answer_text": "(i) An aqueous solution of AgNO3 with silver electrodes.At cathode: Silver ions have lower discharge potential than hydrogen ions. Hence, silver ions ...",
"source":"Toppr",
"domain":"www.toppr.com",
"votes": 0
},
{
"type":"questions_and_answers_element",
"url": "https://www.toppr.com/ask/question/predict-the-products-of-electrolysis-in-each-of-the-followingan-aqueous-solution-of-cucl2with-platinum/",
"question_text":"Predict the products of electrolysis",
"answer_text": "When an aqueous solution of CuCl2 is electrolyzed with platinum electrodes, chlorine is obtained at anode and Cu is deposited at cathode. 2Cl - → Cl2 ...",
"source":"Toppr",
"domain":"www.toppr.com",
"votes": 0
},
{
"type":"questions_and_answers_element",
"url": "https://brainly.in/question/2161527",
"question_text":"Predict the products of electrolysis in each of the following:",
"answer_text": "(i) an aqueous solution of AgNO3 with silver electrode.here two oxidation and two reduction half reactions must be considered .now, oxidation (at anode ...",
"source":"Brainly.in",
"domain":"brainly.in",
"votes": 18
},
{
"type":"questions_and_answers_element",
"url": "https://learn.careers360.com/ncert/question-predict-the-product-of-electrolysis-in-each-of-the-following-an-aqueous-solution-of-cucl2-with-platinum-electrodes/",
"question_text":"Predict the products of electrolysis in each of the following:",
"answer_text":"342",
"source":"Careers360",
"domain":"learn.careers360.com",
"votes": 1
},
{
"type":"questions_and_answers_element",
"url": "https://learn.careers360.com/ncert/question-predict-the-products-of-electrolysis-in-each-of-the-following-a-dilute-solution-of-h2so4-with-platinum-electrodes/",
"question_text":"Predict the products of electrolysis in each of the following:",
"answer_text":"135",
"source":"Careers360",
"domain":"learn.careers360.com",
"votes": 1
}
],
"rectangle": null
}

